Study Definitions
Meta-Analysis
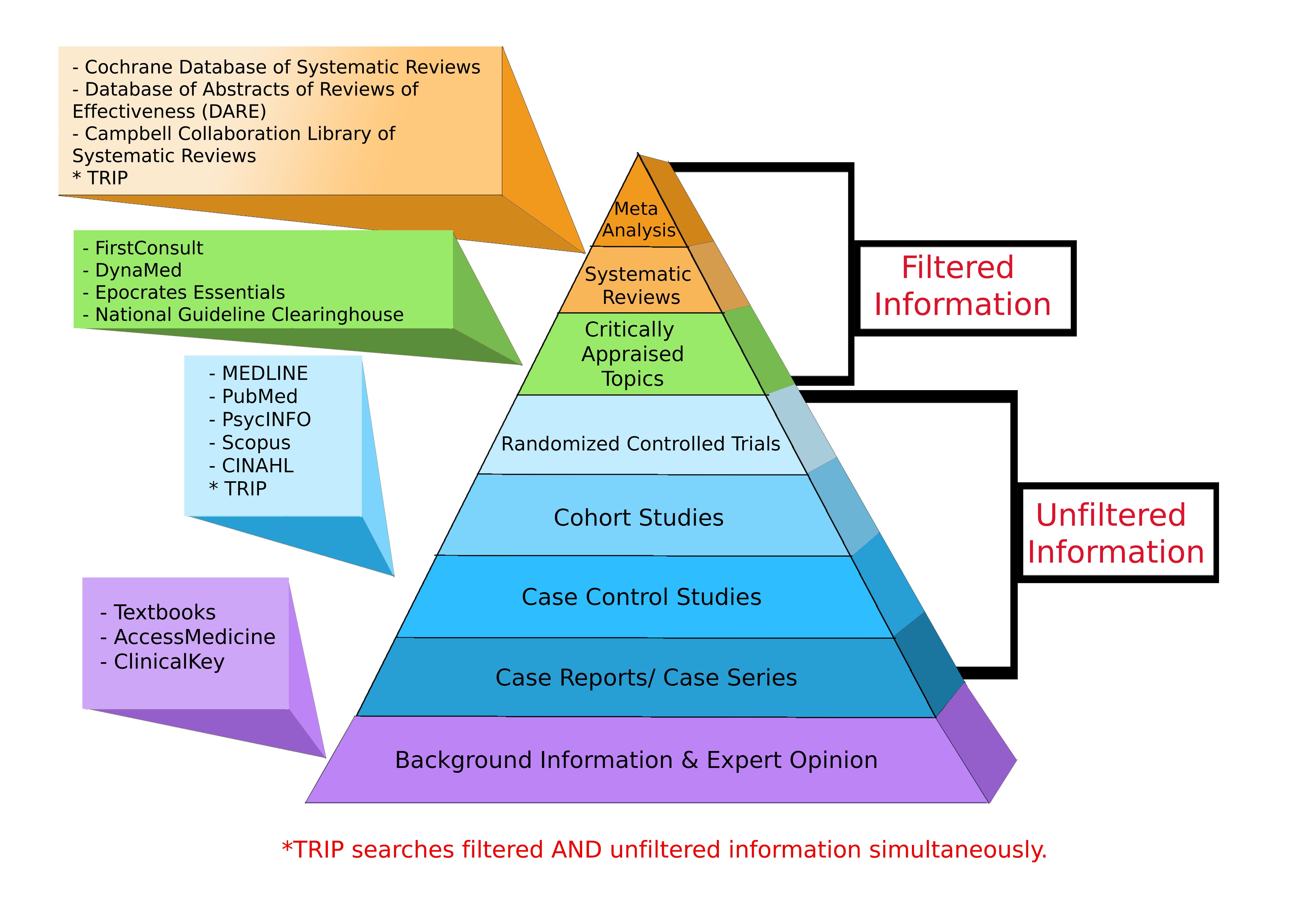
A quantitative method of combining the results of independent studies, which are drawn from the published literature, and synthesizing summaries and conclusions.
Systematic Review
A review which endeavors to consider all published and unpublished material on a specific question. Studies that are judged methodologically sound are then combined quantitatively or qualitatively depending on their similarity.
Randomized Control Trial (RCT)
A clinical trial involving one or more new treatments and at least one control treatment with specified outcome measures for evaluating the intervention. The treatment may be a drug, device, or procedure. Controls are either placebo or an active treatment that is currently considered the "gold standard". If patients are randomized via mathmatical techniques then the trial is designated as a randomized controlled trial.
Cohort Study
In cohort studies, groups of individuals, who are initially free of disease, are classified according to exposure or non-exposure to a risk factor and followed over time to determine the incidence of an outcome of interest. In a prospective cohort study, the exposure information for the study subjects is collected at the start of the study and the new cases of disease are identified from that point on. In a retrospective cohort study, the exposure status was measured in the past and disease identification has already begun.
Case-Control Study
Studies that start by identifying persons with and without a disease of interest (cases and controls, respectively) and then look back in time to find differences in exposure to risk factors.
Cross-Sectional Study
Studies in which the presence or absence of disease or other health-related variables are determined in each member of a population at one particular time.
Levels of Evidence Pyramid
Levels of Evidence Pyramid created by Andy Puro, September 2014
Experimental vs. Observational Studies
An observational study is a study in which the investigator cannot control the assignment of treatment to subjects because the participants or conditions are not being directly assigned by the researcher.
- Examines predetermined treatments, interventions, policies, and their effects
- Four main types: case-series, case-control, cross-sectional, and cohort studies
In an experimental study, the investigators directly manipulate or assign participants to different interventions or environments.
- Experimental studies that involve humans are called clinical trials. They fall into two categories: those with controls, and those without controls.
- Controlled trials - studies in which the experimental drug or procedure is compared with another drug or procedure
- Uncontrolled trials - studies in which the investigators' experience with the experimental drug or procedure is described, but the treatment is not compared with another treatment
Formal Trials versus Observational Studies (Ravi Thadhani, Harvard Medical School)
Study Designs (Centre for Evidence Based Medicine, University of Oxford)
Learn about Clinical Studies (ClinicalTrials.gov, National Institutes of Health)
Definitions taken from: Dawson B, Trapp R.G. (2004). Chapter 2. Study Designs in Medical Research. In Dawson B, Trapp R.G. (Eds), Basic & Clinical Biostatistics, 4e Retrieved September 15, 2014 from http://accessmedicine.mhmedical.com/content.aspx?bookid=356&Sectionid=40086281.



