Experimental vs. Observational Studies
An observational study is a study in which the investigator cannot control the assignment of treatment to subjects because the participants or conditions are not directly assigned by the researcher.
- Examines predetermined treatments, interventions, policies, and their effects
- Four main types: case series, case-control studies, cross-sectional studies, and cohort studies
In an experimental study, the investigators directly manipulate or assign participants to different interventions or environments
Experimental studies that involve humans are called clinical trials. They fall into two categories: those with controls, and those without controls.
- Controlled trials - studies in which the experimental drug or procedure is compared with another drug or procedure
- Uncontrolled trials - studies in which the investigators' experience with the experimental drug or procedure is described, but the treatment is not compared with another treatment
Definitions taken from: Dawson B, Trapp R.G. (2004). Chapter 2. Study Designs in Medical Research. In Dawson B, Trapp R.G. (Eds), Basic & Clinical Biostatistics, 4e. Retrieved September 15, 2014 from https://accessmedicine.mhmedical.com/book.aspx?bookid=2724
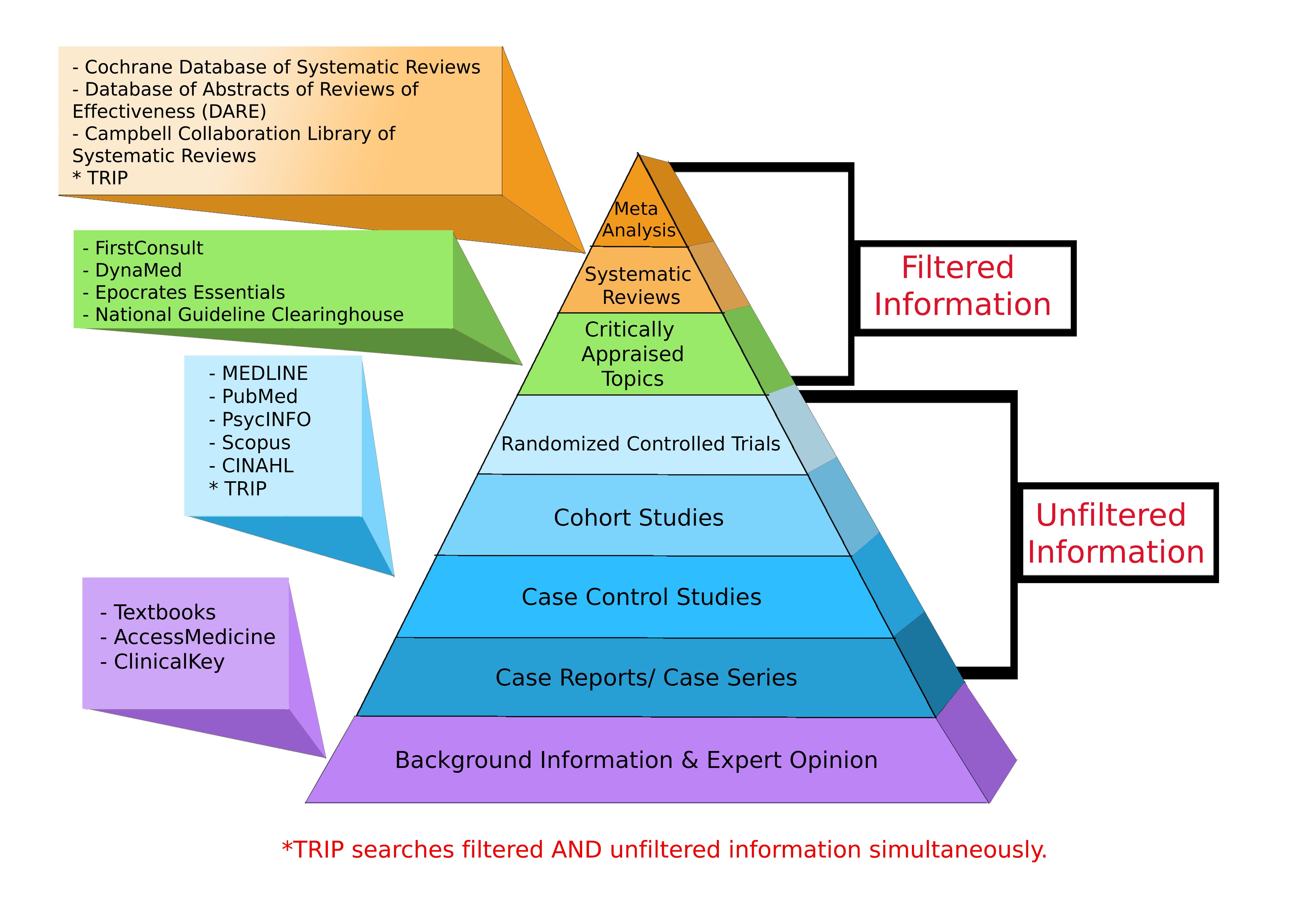
Levels of Evidence Pyramid
Levels of Evidence Pyramid created by Andy Puro, September 2014
Additional Study Design Resources
Study Design 101: Himmelfarb's tutorial on study types and how to find them
Study Designs (Centre for Evidence Based Medicine, University of Oxford)
Learn about Clinical Studies (ClinicalTrials.gov, National Institutes of Health)
Study Designs Guide (Deakin University)




